Commission charts narrow path for editing human embryos: and what about chimaeras?
by
Jon Cohen* and Robert Gorter**, MD, PhD.
September 3, 2020
No recent biomedical experiment has caused more consternation than He Jiankui’s creation of the first gene-edited babies, in 2018, which was widely seen as dangerous, unethical, and premature—and which led to his incarceration by China. Now, an international committee has concluded that gene-editing methods, despite substantial improvements, are still far from mature enough to safely introduce heritable DNA modifications into human embryos. But: the international committee is not against engineering human babies.

He Jiankui shocked the world when he described the implantation of edited embryos that led to the birth of twin girls, Lulu and Nana (CC BY-NC-SA 2.0)
But they will be one day, in rare circumstances, adds the panel, calling for the formation of a global scientific body that would review proposals for what it calls “heritable human genome editing” (HHGE) and try to influence whether countries decide to allow its use. The group, which today released one of the most in-depth reports on the topic yet, spells out in great detail genetic situations that HHGE could address and the strict oversight that clinicians in the future must meet before again creating humans with modified or synthetic DNA that they can pass on to offspring.
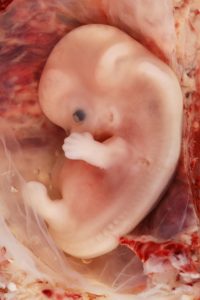
Eight-week old human embryo
For more than 1 year, the International Commission on the Clinical Use of Human Germline Genome Editing reviewed the scientific literature on CRISPR and other ways to modify DNA, held public meetings and webinars, and consulted scientists, physicians, ethicists, and patient groups. The 18 members of the commission—who come from 10 countries and, as the report notes, include “experts in science, medicine, genetics, ethics, psychology, regulation, and law”—agreed with earlier groups that concluded no one should follow in He’s footsteps anytime soon. CRISPR—the genome editor He used, and refined versions of it—they concluded, still cannot “efficiently and reliably” make precise changes without causing “undesired changes in human embryos.”
The report stresses that it focuses on “initial clinical use” of HHGE, and says the field needs to be closely monitored and frequently reevaluated. “There are a lot of gaps in our knowledge and further research is needed,” Kay Davies, a geneticist at the University of Oxford who co-chaired the commission, said at a briefing today.
Organized by the U.K.’s Royal Society and the science and medicine branches of the U.S. National Academies of Sciences, Engineering, and Medicine, the commission aimed to describe a “responsible clinical translational pathway” that could move genome editing from the lab to assisted-reproduction interventions for human diseases. The report largely steers clear of the complex social and ethical implications of creating gene-edited babies. But it does delve into the governance of the issue, notably calling for creating an International Scientific Advisory Panel to assess proposed uses of HHGE, provide regular updates about related technologies, and review clinical outcomes if an edited embryo implanted into a mother is born. It also recommends the creation of an international mechanism by which a clinician or researcher could report plans for or uses of HHGE that they find concerning—in essence, a hotline for whistleblowers.
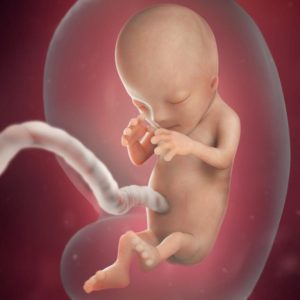
Twelve weeks old human embryo
The commission’s foray into such issues has perturbed some. “The report strays beyond its scientific remit,” Sarah Norcross, director of the Progress Educational Trust, a charity focused on helping people with infertility or genetic conditions, said in a statement released by the U.K. Science Media Centre. “The World Health Organization (WHO) is still deliberating on the governance of genome editing, and should not feel constrained by this report’s governance recommendations if it sees fit to deviate from them.”
The genome editing commission categorized potential uses of HHGE, creating a six-level hierarchy that ranges from the most to least compelling rationales to take the risk. The use of HHGE that is easiest to justify, they said, would be helping those rare couples who, even with in vitro fertilization (IVF) and screening of embryos, have little or no chance of having a baby that does not inherit a genetic condition—for example, Huntington disease, cystic fibrosis, beta thalassemia—that will cause “severe morbidity or premature death.” The report stresses that these situations are few and far between. People have two copies of most genes, one inherited from the mother and the other from the father. For a so-called recessive disorder such as cystic fibrosis, there are maybe one or two couples in the United States who both are “homozygous” for this mutation—meaning in their inherited pair of the gene—and would produce an affected child. In dominant disorders, like Huntington, a child needs to inherit only one mutated gene to develop the disease, so one homozygous parent, also a rarity, inevitably would pass on the disease to all embryos.
If HHGE is allowed, the panel said, any embryo edit should only “specifically change one DNA sequence into a specific desired sequence” that is common in “the relevant population.” This means the simplest, most frequently used form of CRISPR, which can cripple genes but does not fix them, should not ever be used in embryos; in He’s controversial experiment, for example, he attempted to knock out a gene and make the children’s cells resistant to HIV infection.
“I welcome the commission’s report, which continues to add depth to the ongoing global conversation about the science of germline editing,” says Alta Charo, a bioethicist at the University of Wisconsin, Madison, who is part of a committee organized by WHO that is examining how to best govern this controversial arena.
Harvard University chemist David Liu, who has pioneered improved genome editing technologies that borrow from CRISPR’s toolkit, describes the report as “thoughtful, balanced, and well-bounded.” But he still has misgivings about whether HHGE should ever be allowed. “I continue to struggle to imagine plausible situations in which clinical germline editing provides a path forward to address an unmet medical need that cannot be provided by other options,” Liu says. He and others stress that preimplantation genetic testing (PGT), which IVF clinics routinely use, could avoid the need for most human embryo editing. In all but the rarest circumstances, it would allow couples to select and then implant embryos that did not have the disease-causing mutations borne by parents.
There are some couples, however, who have a high likelihood of PGT failing to give them an unaffected child, and this is the one exception to the second-tiered category in the report. The third category of HHGE uses is for genetic diseases that have less serious effects and may also be corrected or treated, like deafness, for which there are now cochlear implants.
In the wake of He’s 2018 revelation, Denis Rebrikov, a DNA sequencing specialist at the Pirogov Russian National Research Medical University, has pursued a project to correct a deafness mutation in couples who each have the aberrant gene. Rebrikov, who is not yet satisfied he can safely edit a human embryo and so has not sought Russia’s approval to move forward, says the cases for which the commission would allow HHGE are so rare that the panel’s endorsement is meaningless. “In this formulation, it is a ban on editing the genome of the embryo in principle,” Rebrikov says.
Norcross echoes that criticism, calling the report’s criteria for human embryo editing “far too narrow.”
Diseases caused by several genes represent the fourth category of HHGE uses. The fifth, and most taboo in the eyes of the panel, would involve genetic enhancements of children, making make them resistant to HIV, better at sports, taller, smarter, or even able to withstand radiation exposures encountered during extended spaceflight or nuclear wars.
A key danger of editing human embryos is that unintended “off-target” DNA changes will occur and not be detected before embryo implantation. The panel explores in detail a possible solution: editing the stem cells that produce human sperm or eggs before using those gametes for IVF. “This would have significant safety implications since the issues of on-target editing fidelity and avoidance of off-target events could be largely settled before any gamete is considered for use in the creation of an embryo,” the report notes.
Fyodor Urnov, a CRISPR researcher at the University of California, Berkley, says the report confirms the widespread consensus that, at best, there’s only a niche justification for editing human embryos. “The careful guidelines laid out in this report show that the list of problems that could be addressed by such editing is, in fact, quite small,” Urnov says. “It is an open secret in the gene-editing community that human reproductive editing is a solution in search of a problem.”
The He Jiankui affair is a scientific and bioethical circumstance concerning the use of gene-editing in human cases following the first use by Chinese scientist He Jiankui, who made the first genome-edited human babies in 2018.[1][2] The affair led to legal and ethical controversies with an indictment of He and his two collaborators, Zhang Renli and Qin Jinzhou.
He Jiankui, working at the Southern University of Science and Technology (SUSTech) in Shenzhen, China, started a project to help people with fertility problems, specifically involving HIV-positive fathers and HIV-negative mothers. The subjects were offered standard in vitro fertilization services and in addition, use of CRISPR gene editing (CRISPR/Cas9), a technology for modifying DNA. Specifically, the embryos were edited of their CCR5 gene in an attempt to confer genetic resistance to HIV.[3] The clinical project was conducted secretly until 25 November 2018 when MIT Technology Review exposed the story about the human experiment based on information from the Chinese clinical trials registry. Compelled by the situation, He immediately announced the birth of genome-edited babies in a series of five videos on YouTube the same day.[4][5] The first babies, known by their pseudonyms Lulu (Chinese: 露露) and Nana (Chinese: 娜娜), are twin girls born in October 2018, and the second birth or the third baby born was in 2019.[6][7][8] He reported that the babies were born healthy.[9]
The reaction to He’s actions was widespread criticism[10][11] and included concern for the well-being of the girls.[3][12][13] He presented his research at the Second International Summit on Human Genome Editing at the University of Hong Kong on 28 November 2018. The next day, Chinese authorities suspended all his research activities.[14] He was immediately detained in SUSTech campus and kept under surveillance. On 30 December 2019, Chinese authorities announced that he was found guilty of forging documents and unethical conduct; he was sentenced to three years in prison with a fine of 3 million yuan (US$430,000).[15][16]
As a consequence to He’s work, the World Health Organization launched a global registry in 2019 to track research on human genome editing, after a call to halt all work on genome editing.[17][18][19] In May 2019, lawyers in China reported, in light of He’s experiment, the drafting of regulations that anyone manipulating the human genome by gene-editing techniques, like CRISPR, would be held responsible for any related adverse consequences.[20]
He Jiankui has been variously referred to as a “rogue” scientist,[6] “China’s Dr Frankenstein”,[21] and a “mad genius”.[22]
https://www.sciencemag.org/…/did-crispr-help-or-harm-first-ever-gene-edited-babies
A related field to genetic manipulation is growing creatures that are in-part animal and in-part human. Laboratories around the world are experimenting and growing in laboratories and on farms, chimeras for the organ transplant industry.
“We can make an animal without a heart. We have engineered pigs that lack skeletal muscles and blood vessels,” says Daniel Garry, a cardiologist who leads a chimera project at the University of Minnesota. While such pigs aren’t viable, they can develop properly if a few cells are added from a normal pig embryo. Garry says he’s already melded two pigs in this way and recently won a $1.4 million grant from the U.S. Army, which funds some biomedical research, to try to grow human hearts in swine.
“The specter of an intelligent mouse stuck in a laboratory somewhere screaming ‘I want to get out’ would be very troubling to people.”
Should the state (taxpayers) have to foot the bill for endless organ transplants for people who choose to live unhealthy lifestyles? And what if legislating bodies say they don’t want to cover unlimited transplants for drug abusers or criminals, can they not be accused of “literally killing people” for denying them health insurance coverage for organ transplants?
Will the practice of medicine now involve the mass murder of animals in order to supply a steady stream of transplant organs?

Could this be one of the very first dog-human chimeras in 2023?
Coming up next:
Human babies grown in labs and sacrificed for organ and stem cell harvesting and embryonic tissues for the development of new vaccines and then, their mass production. Currently, more than half of all vaccines applied were cultivated in human aborted stem (diploid) cells.
The organ transplant industry is steeped in medical ethics violations and the mass murder of conscious beings. It won’t be long before the industry will want to genetically engineer human babies to be grown for organ harvesting.

Injecting cells from one species into the embryo of another creates mixtures called chimeras. From left to right: an ordinary mouse, a mouse that’s partly rat, a rat that’s partly mouse, a white rat.
Posted in: Health
doi:10.1126/science.abe6341
References
1: Greely, Henry T (2019). “CRISPR’d babies: human germline genome editing in the ‘He Jiankui affair'”. Journal of Law and the Biosciences. 6 (1): 111–183. doi:10.1093/jlb/lsz010. PMC 6813942. PMID 31666967.
2: Cyranoski, David (22 January 2019). “CRISPR-baby scientist fired by university”. Nature. doi:10.1038/d41586-019-00246-2. Retrieved 9 January 2020.
3: “China Orders Investigation After Scientist Claims First Gene-Edited Babies”. The New York Times. Reuters. 26 November 2018. Archived from the original on 27 November 2018. Retrieved 26 November 2018.
4: Regalado, Antonio (25 November 2018). “Chinese scientists are creating CRISPR babies”. MIT Technology Review.
5: Bulluck, Pam (14 April 2019). “Gene-Edited Babies: What a Chinese Scientist Told an American Mentor”. The New York Times. Retrieved 14 April 2019.
6: Cohen, Jon (2 August 2019). “Inside the circle of trust”. Science. 365 (6452): 430–437. Bibcode:2019Sci…365..430C. doi:10.1126 / science. 365.6452.430. / PMID 31371593.
7: Begley, Sharon; Joseph, Andrew (17 December 2018). “The CRISPR shocker: How genome-editing scientist He Jiankui rose from obscurity to stun the world”. Stat News. Retrieved 17 December 2018.
8: Begley, Sharon (26 November 2018). “Claim of CRISPR’d baby girls stuns genome editing summit”. Stat News. Archived from the original on 27 November 2018. Retrieved 26 November 2018.
9: Begley, Sharon (28 November 2018). “Amid uproar, Chinese scientist defends creating gene-edited babies – STAT”. STAT.
10: Kolata, Gina; Belluck, Pam (5 December 2018). “Why Are Scientists So Upset About the First Crispr Babies? – Only because a rogue researcher defied myriad scientific and ethical norms and guidelines. We break it down”. The New York Times. Retrieved 5 December 2018.
11: The Editorial Board (28 January 2019). “Should Scientists Toy With the Secret to Life? – The gene-editing technology Crispr has the power to remake life as we know it. Questions about how to use it concern everyone”. The New York Times. Retrieved 29 January 2019.
12: Regalado, Antonio (25 November 2018). “Exclusive: Chinese scientists are creating CRISPR babies – A daring effort is under way to create the first children whose DNA has been tailored using gene editing”. MIT Technology Review. Archived from the original on 27 November 2018. Retrieved 26 November 2018.
13: Cyranoski, David (27 November 2018). “How the genome-edited babies revelation will affect research – Some scientists worry the startling claim will lead to knee-jerk regulations and damage the public’s trust in gene editing”. Nature. doi:10.1038/d41586-018-07559-8. Archived from the original on 27 November 2018. Retrieved 27 November 2018.
14: Jiang, Steven; Regan, Helen; Berlinger, Joshua (29 November 2018). “China suspends scientists who claim to have produced first gene-edited babies”. CNN News. Retrieved 29 November 2018.
15: Wee, Sui-Lee (30 December 2019). “Chinese Scientist Who Genetically Edited Babies Gets 3 Years in Prison – He Jiankui’s work was also carried out on a third infant, according to China’s state media, in a new disclosure that is likely to add to the global uproar over such experiments”. The New York Times. Retrieved 30 December 2019.
16: Yee, Isaac; Hollingsworth, Julia (30 December 2019). “Chinese gene-editing scientist jailed for 3 years”. CNN News. Retrieved 30 December 2019.
17: “The World Health Organization Says No More Gene-Edited Babies”. WIRED. 30 July 2019. Retrieved 26 November 2019.
18: “WHO To Create Registry for Genetic Research”. Voice of America. 29 August 2019. Retrieved 26 November 2019.
19: “The WHO panel calls for registry of all human gene editing research”. Reuters. 20 March 2019. Retrieved 26 November 2019.
20: Cyranoski, David (20 May 2019). “China set to introduce gene-editing regulation following CRISPR-baby furore – The draft rules mean that anyone who manipulates human genes in adults or embryos is responsible for adverse outcomes”. Nature. doi:10.1038/d41586-019-01580-1. PMID 32424191. Retrieved 20 May 2019.
21: Yan, Sophia (28 November 2018). “China’s ‘Dr Frankenstein’ says second woman in early pregnancy with gene-edited babies”. The Telegraph. Retrieved 10 January 2020.
22: Low, Zoe (27 November 2018). “China’s gene editing Frankenstein had dreams of being Chinese Einstein”. South China Morning Post. Retrieved 10 January 2020